Project 01
Insights into lipopolysaccharide (LPS) transport by solid-state NMR spectroscopy
Principle Investigator: Prof. Dr. Clemens Glaubitz
Research Areas: Membrane Biophysics, NMR Spectroscopy
Summary
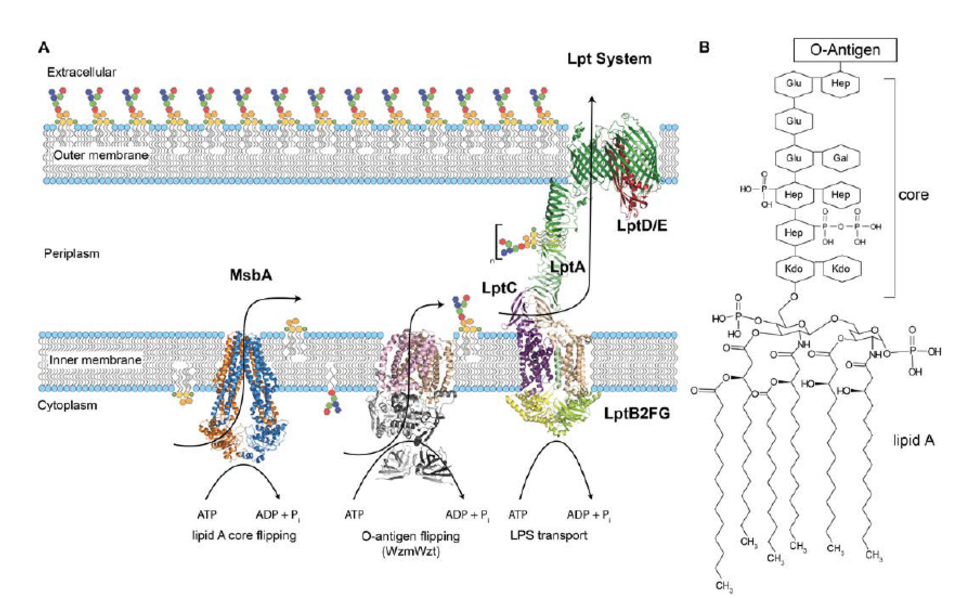
The cell envelope of Gram-negative bacteria consists of an inner membrane with a symmetric phospholipid bilayer, the periplasm containing the peptidoglycan layer, and the asymmetric outer membrane. Its outer leaflet is mainly comprised of lipopolysaccharides (LPS), while the inner leaflet contains the same lipids as found in the innermembrane. Maintaining this unique asymmetry is an essential mechanism of protecting the bacteria against antibiotics or the mammalian immune system. LPS is synthesized in the cytosolic leaflet of the inner membrane. The ATP-binding cassette (ABC) transporter MsbA flips LPS over to the periplasmic leaflet. LPS is extracted from thereby an assembly of seven LPS transport (Lpt) proteins to be inserted into the outer leaflet of the outer membrane via a molecular ‘lipid bridge’. This bridge consists of the ABC transporter complex LptB2FG/C in the inner membraneand the lipoprotein b-barrel LptE/D in the outer membrane. The soluble protein LptA bridges both membrane protein complexes. The mechanisms of LPS flipping by MsbA in the inner membrane and its transfer by the Lpt-complex to the outer membrane have not been fully resolved yet. We will use solid-state NMR to elucidate theinteraction of LPS with all of these proteins, starting with MsbA and LptB2FG, followed by LptC/A and subsequently with LptE/D. Our approaches include multidimensional high-field MAS-NMR techniques as well as dynamic nuclear polarization to boost sensitivity by orders of magnitude. All NMR experiments will be conducted inliposomes and complemented by measurements on native membrane preparations under in situ conditions. Within the CRC, a very strong thematic overlap and synergy is given by the work on ABC transporters (with Joseph, P02 and Tampé, P18) as well as dynamic assembly and transport machineries bridging the inner and outer bacterialmembrane (with Joseph, P02 and Pos, P03). Our solid-state NMR approach will also be used to probe conformational changes in the ion channel YugO (Hänelt, P04), the lipid transporters MlaA and CitA (Pos, P03) andfor the functional dynamics of FGF receptors in the membrane (Schwalbe, P10). Furthermore, expertise on photoreceptors will be provided (Wachtveitl, P05).

Prof. Dr. Clemens Glaubitz
Steering Board of the CRC 1507
Institute of Biophysical Chemistry
Goethe University, Frankfurt
P01: PROJECT-RELATED PUBLICATIONS
- Klausnitzer A, Kaur J, Rath T, Becker-Baldus J, Morgner N, Glaubitz C (2025) Conformational plasticity of LptC regulates lipopolysaccharide transport by the LptB2FGC complex. J Am Chem Soc, accepted
- Novischi SYP, Karoly-Lakatos A, Chok K, Bonifer J, Becker-Baldus C, Glaubitz C (2024) Probing the allosteric NBD-TMD crosstalk in the ABC transporter MsbA by solid-state NMR. Commun Biol 7: 43
- Maciejko J, Kaur J, Becker-Baldus J, Glaubitz C (2019) Photocycle-dependent conformational changes in the proteorhodopsin cross-protomer Asp-His-Trp triad revealed by DNP-enhanced MAS-NMR. Proc Natl Acad Sci USA 116: 8342-8349
- Möbius K, Kazemi S, Güntert P, Jakob A, Heckel A, Becker-Baldus J, Glaubitz C (2019) Global response of diacylglycerol kinase towards substrate binding observed by 2D and 3D MAS NMR. Sci Rep 9: 3995
- Mao J, Aladin V, Jin X, Leeder AJ, Brown LJ, Brown RCD, He X, Corzilius B, Glaubitz C (2019) Exploring protein structures by DNP-enhanced methyl solid-state NMR spectroscopy. J Am Chem Soc 141: 19888-19901
- Spadaccini R, Kaur H, Becker-Baldus J, Glaubitz C (2018) The effect of drug binding on specific sites in transmembrane helices 4 and 6 of the ABC exporter MsbA studied by DNP-enhanced solid-state NMR. Biochim Biophys Acta 1860: 833-840
- Kaur H, Abreu B, Akhmetzyanov D, Lakatos-Karoly A, Soares CM, Prisner T, Glaubitz C (2018) Unexplored nucleotide binding modes for the ABC exporter MsbA. J Am Chem Soc 140: 14112-14125
- Joedicke L, Mao J, Kuenze G, Reinhart C, Kalavacherla T, Jonker HRA, Richter C, Schwalbe H, Meiler J, Preu J, Michel H, Glaubitz C (2018) The molecular basis of subtype selectivity of human kinin G-protein-coupled receptors. Nat Chem Biol 14: 284- 290
- Lehnert E, Mao J, Mehdipour AR, Hummer G, Abele R, Glaubitz C, Tampe R (2016) Antigenic peptide recognition on the human ABC transporter TAP resolved by DNP- enhanced solid-state NMR spectroscopy. J Am Chem Soc 138: 13967-13974
- Kaur H, Lakatos-Karoly A, Vogel R, Noll A, Tampe R, Glaubitz C (2016) Coupled ATPase-adenylate kinase activity in ABC transporters. Nat Commun 7: 13864
