Project 04
Regulation of potassium channel YugO in bacterial electrical
Principle Investigator: Inga Hänelt
Research Areas: Biochemistry, Microbiology, Structural Biology
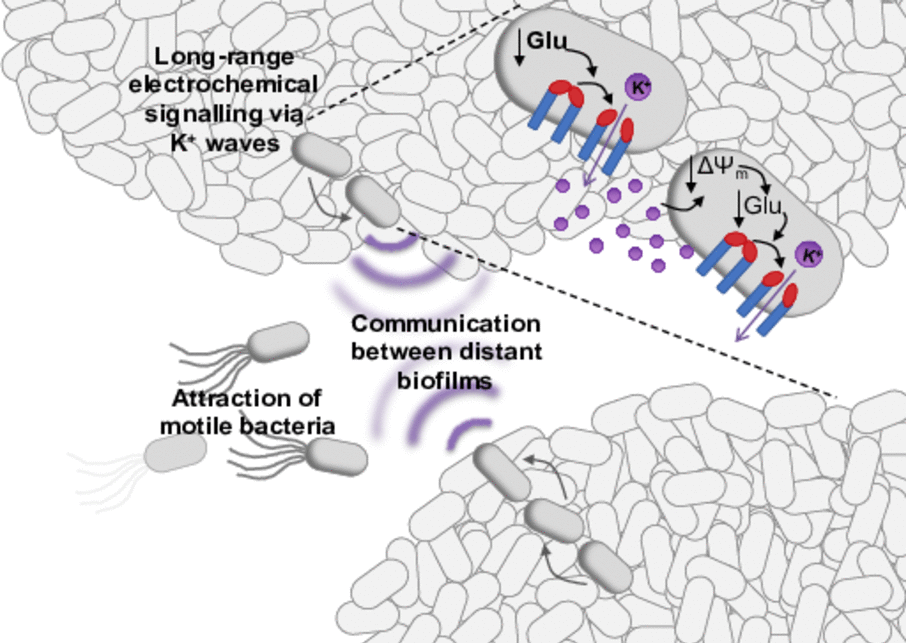
Summary
Neurons have long been known to use electrical signaling for long-range communication. Recently, prokaryotes showed an important comparable function: in bacterial biofilms potassium ion channels mediate electrical signal transduction, which serves cell-cell communication allowing the communication within a biofilm, the synchronizationof two or more biofilms with each other and the attraction of motile bacteria to a biofilm. Interestingly, the electrical signaling even permits interspecies interaction, since potassium ions are essential currencies for all cells. Thus, electrical signaling might be much more universal than the well- described chemical signaling in biofilms via quorum sensing. The key player for electrical signaling in Bacillus subtilis is potassium ion channel YugO. Upon glutamate starvation within the interior cells of a biofilm, YugO supposedly opens leading to the efflux of potassium ions. The resulting hyperpolarization of the interior cells favors the uptake of glutamate while the more peripheral,depolarized cells consume less glutamate. Following a domino effect or chain reaction, the electrical signalpropagates through the biofilm enabling the diffusion of glutamate deep into the interior. This way, the survival of the whole bacterial community is assured. Biofilm formation is a characteristic of most multidrug-resistant bacteria. Disturbing the electrical signaling might thus be a new antibiotic strategy. However, to develop such a strategy molecular details are required. By microbial cell biology, structural biology, and biophysical approaches we will unravel the foundation of bacterial electrical signaling. Exemplarily for B. subtilis we will be able to answer how ion channel YugO gets activated on a molecular level, and which stimuli are responsible for the initiation of the signaling and for the propagation through the biofilm.

Inga Hänelt
Steering Board of the CRC 1507 & Head of IRTG
Institute of Biochemistry Goethe University, Frankfurt
04: PROJECT-RELATED PUBLICATIONS
- Tascon I, Sousa J, Corey RA, Mills DJ, Griwatz D, Aumüller N, Mikusevic V, Stansfeld PJ, Vonck J*, Hänelt I* (2020) Structural basis of proton-coupled potassium transport in the KUP family. Nat Commun 11: 626
- Mikusevic V, Schrecker M, Kolesova N, Patino-Ruiz M, Fendler K, Hänelt I (2019) A channel profile report of the unusual K+ channel KtrB. J Gen Physiol 151: 1357-1368
- Stock C, Hielkema L, Tascon I, Wunnicke D, Oostergetel G, Azkargorta M, Paulino C*, Hänelt I* (2018) Cryo-EM structures of KdpFABC suggest a K+ transport mechanism via two inter-subunit half-channels. Nat Commun 9: 4971
- Diskowski M, Mehdipour AR, Wunnicke D, Mills DJ, Mikusevic V, Bärland N, Hoffmann J, Morgner N, Steinhoff HJ, Hummer G, Vonck J*, Hänelt I* (2017) Helical jackknives control the gates of the double-pore K+ uptake system KtrAB. eLife 6: e24303
- Hänelt I, Jensen S, Wunnicke D, Slotboom DJ (2015) Low affinity and slow Na+ binding precedes high affinity aspartate binding in GltPh. J Biol Chem 290: 15962-15972
- Shiyan A, Thompson M, Köcher S, Tausendschön M, Santos H, Hänelt I, Müller V (2014) Glutamine synthetase 2 is not essential for biosynthesis of compatible solutes in Halobacillus halophilus. Front Microbiol 5: 168
- Erkens GB, Hänelt I, Goudsmits JMH, Slotboom DJ, van Oijen AM (2013) Unsynchronised subunit motion in single trimeric sodium-coupled aspartate transporters. Nature 502: 119-123
- Jensen S, Guskov A, Rempel S, Hänelt I, Slotboom DJ (2013) Crystal structure of a substrate-free aspartate transporter. Nat Struct Mol Biol 20: 1224-1226
- Hänelt I, Wunnicke D, Bordignon E, Steinhoff HJ, Slotboom DJ (2013) Conformational heterogeneity of the aspartate transporter Glt(Ph). Nat Struct Mol Biol 20: 210-214
- Hänelt I*, Löchte S, Sundermann L, Elbers K, Vor der Brüggen M, Bakker EP (2010) Gain of function mutations in membrane region M2C2 of KtrB open a gate controlling K+ transport by the KtrAB system from Vibrio alginolyticus. J Biol Chem 285: 10318-10327
