Project 14
Structural basis of complex I function and assembly
Principle Investigator: Dr. Janet Vonck, Prof. Dr. Volker Zickermann
Research Areas: Structural Biology and Biochemistry
Summary
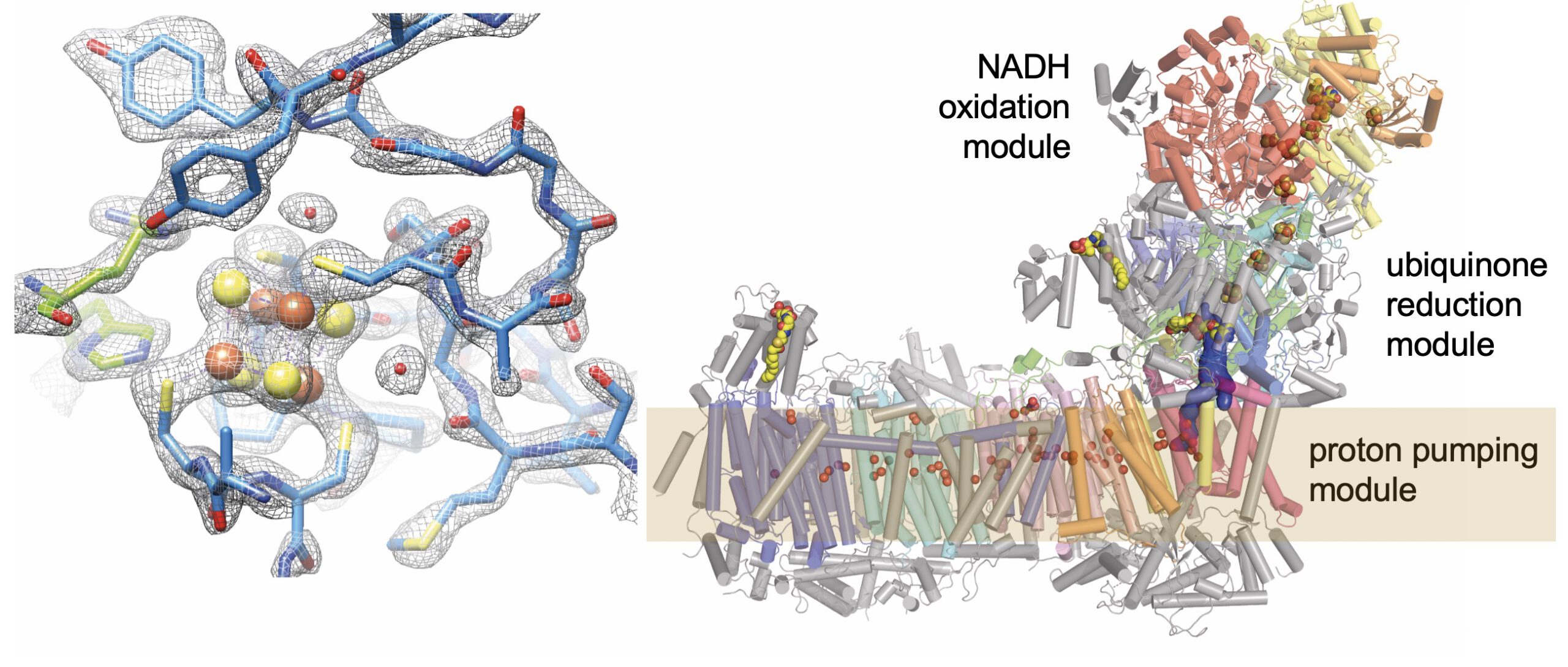
Respiratory complex I is a 1 MDa membrane protein complex with a central role in energy metabolism. In humans, assembly of the intricate molecular machine (44 subunits, 9 redox active cofactors) requires more than 15 assembly factors. We use complex I from the aerobic yeast Yarrowia lipolytica as a model system. Our high-resolution cryo-EM structures of complex I in native form and during steady state activity have enabled us to define proton translocation pathways by protein-bound water molecules and to monitor detailed conformational changes during turnover. Our first goal is to gain a comprehensive understanding of the catalytic mechanism of complex I. Cryo-EM structures of assembly intermediates have helped us elucidate the role of NDUFAF1 in building the proton pumping module and of NDUFAF2 in attaching the NADH oxidation module. Our second goal is to understand complex I assembly and the function of the assembly factors in each step. Our work on the structure, function and assembly of complex I has offered clues on the pathogenesis of NDUFS4-linked Leigh syndrome. Our third goal is to take advantage of our yeast genetic model system to unravel how complex I dysfunction causes human diseases.
P14: PROJECT-RELATED PUBLICATIONS
- Schiller J, Laube E, Wittig I, Kühlbrandt W, Vonck J, Zickermann V (2022) Insights into complex I assembly: Function of NDUFAF1 and a link with cardiolipin remodeling. Sci Adv 8: eadd3855
- Parey K, Lasham J, Mills DJ, Djurabekova A, Haapanen O, Galemou Yoga E, Xie H, Kühlbrandt W, Sharma V, Vonck J, Zickermann V (2021) High-resolution structure and dynamics of mitochondrial complex I – insights into the proton pumping mechanism. Sci Adv 7: abj3221
- Galemou Yoga E, Parey K, Djurabekova A, Haapanen O, Siegmund K, Zwicker K, Sharma V, Zickermann V, Angerer H (2020) Essential role of accessory subunit LYRM6 in the mechanism of mitochondrial complex I. Nat Commun 11: 6008
- Parey K, Wirth C, Vonck J, Zickermann V (2020) Respiratory complex I — structure, mechanism and evolution. Curr Opin Struct Biol 63: 1-9
- Parey K, Haapanen O, Sharma V, Köfeler H, Züllig T, Prinz S, Siegmund K, Wittig I, Mills DJ, Vonck J, Kühlbrandt W, Zickermann V (2019) High-resolution cryo-EM structures of respiratory complex I: Mechanism, assembly, and disease. Sci Adv 5: eaax9484
- Parey K, Brandt U, Xie H, Mills DJ, Siegmund K, Vonck J, Kühlbrandt W, Zickermann V (2018) Cryo-EM structure of respiratory complex I at work. eLife 7: e39213
- Cabrera-Orefice A, Yoga EG, Wirth C, Siegmund K, Zwicker K, Guerrero-Castillo S, Zickermann V, Hunte C, Brandt U (2018) Locking loop movement in the ubiquinone pocket of complex I disengages the proton pumps. Nat Commun 9: 4500
- Kahlhofer F, Kmita K, Wittig I, Zwicker K, Zickermann V (2017) Accessory subunit NUYM (NDUFS4) is required for stability of the electron input module and activity of mitochondrial complex I. Biochim Biophys Acta 1858: 175-81
- Kmita K, Wirth C, Warnau J, Guerrero-Castillo S, Hunte C, Hummer G, Kaila VR, Zwicker K, Brandt U, Zickermann V (2015) Accessory NUMM (NDUFS6) subunit harbors a Zn-binding site and is essential for biogenesis of mitochondrial complex I. Proc Natl Acad Sci USA 112: 5685-90
- Zickermann V, Wirth C, Nasiri H, Siegmund K, Schwalbe H, Hunte C, Brandt U (2015) Mechanistic insight from the crystal structure of mitochondrial complex I. Science 347: 44-9


