Project 05
Molecular dynamics of light responsive molecular machines
Principle Investigator: Prof. Dr. Josef Wachtveitl
Research Areas: Biophysics, Physical Chemistry, Molecular Spectroscopy
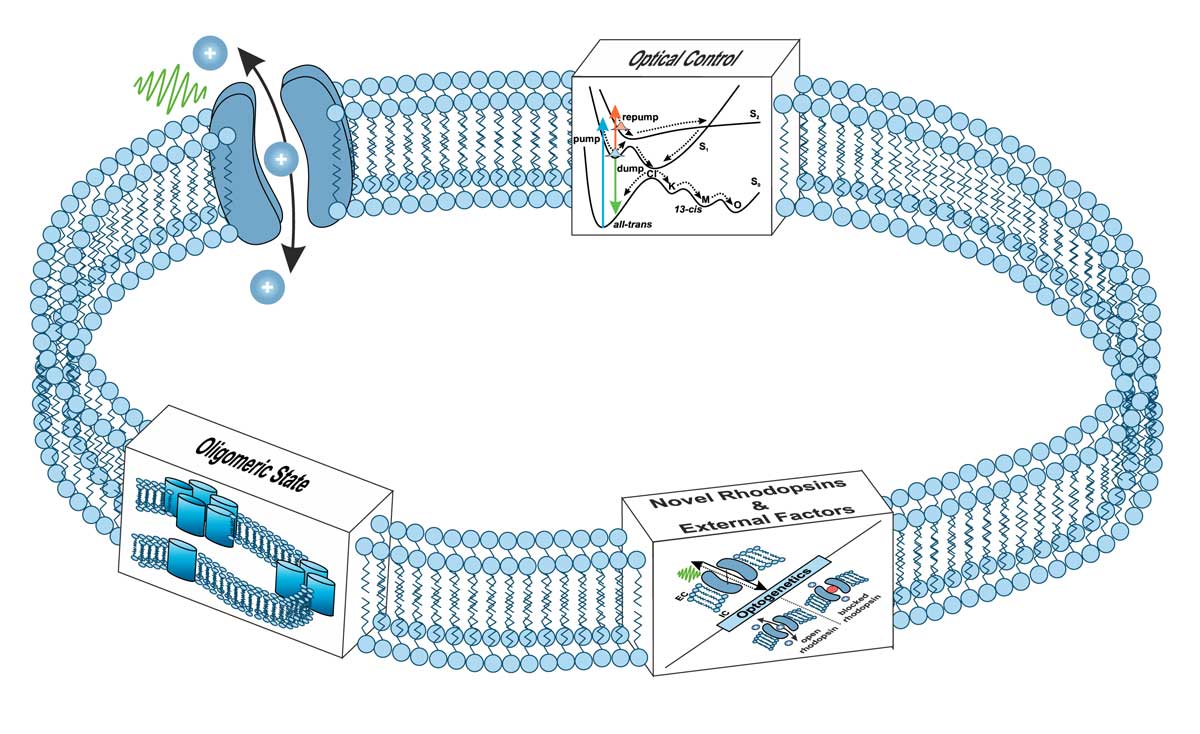
Summary
In this project, we will study conformational dynamics in membrane-associated machineries with a focus on microbial rhodopsins using time-resolved UV/vis and IR spectroscopy. For novel rhodopsins, various spectroscopic methods, covering several orders of magnitude in time, will be applied to track the complete reaction sequence from ultrafast local perturbation to slower long range conformational changes. Furthermore, the impact of external factors (pH, ions, environment, …) on the dynamics and function of novel and well-characterized microbial rhodopsins will be studied together with Glaubitz P01. The presence of certain ions can have large effects on microbial rhodopsins, both structurally and dynamically. Additionally, several microbial rhodopsins have a distinct oligomeric state that is critical for their function and dependent on the physiological environment. Studying the oligomeric state dependence of the complete reaction sequence is another important part of P05 and will lead to further understanding the functional role of supramolecular assemblies of microbial rhodopsins. In addition, we plan to control the function of well-characterized microbial rhodopsins via multipulse experiments. The aim is to go beyond the well-established blue-light quenching and to find additional ways to gain optical control by manipulating the photocycles of microbial rhodopsins in a target manner.

Prof. Dr. Josef Wachtveitl
05: PROJECT-RELATED PUBLICATIONS
- Podoliak E, Lamm GHU, Marin E, Schellbach AV, Fedotov A, Stetsenko A, Asido M, Maliar N, Bourenkov G, Balandin T, Baeken C, Astashkin R, Schneider TR, Bateman A, Wachtveitl J, Schapiro I, Busskamp V, Guskov A, Gordeliy V, Alekseev A, Kovalev K (2024) A Subgroup of Light-driven Sodium Pumps with an Additional Schiff Base Counterion. Nat. Commun. 15, 3119
- Lamm GHU, Zabelskii D, Balandin T, Gordeliy V, Wachtveitl J (2024) Calcium-Sensitive Microbial Rhodopsin VirChR1: A Femtosecond to Second Photocycle Study. J. Phys. Chem. Lett. 15, 5510-5516
- Asido M, Boumrifak C, Weissbecker J, Bamberg E, Wachtveitl J (2024) Vibrational Study of the Inward Proton Pump Xenorhodopsin NsXeR: Switch Order Determines Vectoriality. J. Mol. Biol. 168447: 0022-2836
- Bühl E, Realer T, Lam R, Asido M, Bamberg E, Schlesinger R, Bamann C, Heberle J, Wachtveitl J (2023) Assessing the Role of R120 in the Gating of CrChR2 by Time-Resolved Spectroscopy from Femtoseconds to Seconds. J. Am. Chem. Soc. 145 (40): 2182-21840
- Asido M and Wachtveitl J (2023) Photochemistry of the Light-Driven Sodium Pump Krokinobacter eikastus Rhodopsin 2 and its Implications on Microbial Rhodopsin Research: Retrospective and Perspective. J. Phys. Chem. B: 3766-3773
- Asido M, Kar RK, Kriebel CN, Braun M, Glaubitz C, Schapiro I, Wachtveitl J (2021) Transient Near-UV Absorption of the Light-Driven Sodium Pump Krokinobacter Eikastus Rhodopsin 2: A Spectroscopic Marker for Retinal Configuration. J. Phys. Chem. Lett. 12: 6284-6291
- Weissbecker J, Boumrifak C, Breyer M, Wießalla T, Shevchenko V, Mager T, Slavov C, Alekseev A, Kovalev K, Gordeliy V, Bamberg E, Wachtveitl J (2021) The Voltage Dependent Sidedness of the Reprotonation of the Retinal Schiff Base Determines the Unique Inward Pumping of Xenorhodopsin. Angew Chemie – Int Ed 60: 23010–23017
- Jakdetchai O, Eberhardt P, Asido M, Kaur J, Kriebel CN, Mao J, Leeder AJ, Brown LJ, Brown RCD, Becker-Baldus J, Bamann C, Wachtveitl J, Glaubitz C (2021) Probing the photointermediates of light-driven dodium ion pump KR2 by DNP-enhanced solid-state NMR. Sci Adv 7: 4213–4225
- Asido M, Eberhardt P, Kriebel CN, Braun M, Glaubitz C, Wachtveitl J (2019) Time-resolved IR spectroscopy reveals mechanistic details of ion transport in the sodium pump Krokinobacter eikastus rhodopsin 2. Phys Chem Chem Phys 21: 4461–4471
- Bühl E, Eberhardt P, Bamann C, Bamberg E, Braun, M, Wachtveitl J (2018) Ultrafast protein response in channelrhodopsin-2 studied by time-resolved infrared spectroscopy. J Phys Chem Lett 9: 7180–7184
