Project 10
Modulation of tyrosine receptor signaling investigated by NMR spectroscopy
Principle Investigator: Prof. Dr. Harald Schwalbe
Research Areas: Chemical Biology, NMR Spectroscopy, Organic Chemistry
Summary
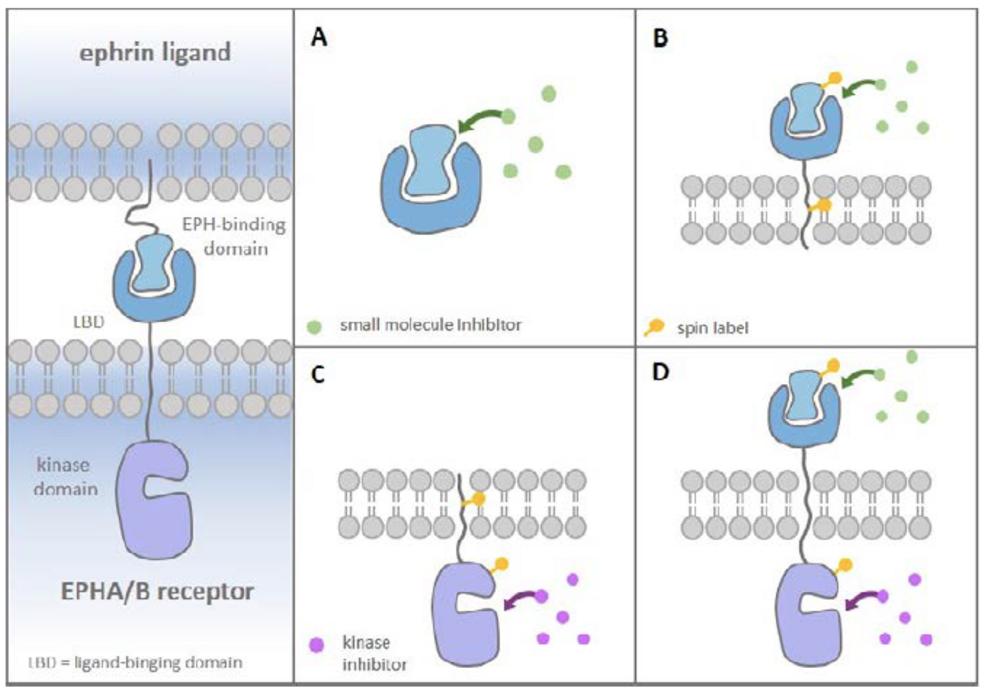
The aim of the project is to investigate tyrosine receptor signaling for two pharmacological systems: the FGF-FGFR receptor and the EPH-Ephrin-receptor. In both systems, we develop new inhibitors of low molecular weight as chemical probes and towards the development of new pharmaceutical active compounds. The FGF/FGFR receptor system is of particular interest for us, as previous studies have shown that the small molecular inhibitor, SSR128129E (SSR), acts allosterically on the extracellular domains (ECDs) of FGFR. Crystal structures of different FGF/FGFR complexes are already available. In order to investigate the dynamics of FGF/FGFR interactions and the influence of small molecular compounds within the CRC 1507, we will characterize the structure in solution by NMR. Since the influence of the transmembrane domain on the FGF/FGFR binding dynamics is not well understood, the TM domain has been expressed in connection with either the FGFR ectodomains or the intracellular tyrosine kinase domain. Reconstitution of the proteins in liposomes (in collaboration with Glaubitz, P01) will allow us to study the receptor structure in a more native- like environment. We will express and purify FGF mutants, which will be used to study the dimerization dynamics in dependence of these FGFs using advanced light microscopy (in collaboration with Heilemann, P08). The EPH/ephrin system is characterized by high complexity: Both EPH receptors and the ligands have to be clustered to trigger efficient activation and, upon activation, tetramers of receptors (homo and hetero EPH subtypes) and ligands assemble to form large signaling arrays. These interactions can induce bidirectional signaling cascades in the participating cells. Within CRC 1507, we will extend our research focus from development of inhibitors targeting the kinase domain to the extracellular domains of EPH, their ligands, the ephrins, and to the transmembrane (TM) domain to discover small molecular compounds or peptides, which can modulate this biomedically relevant protein- protein interaction using structural biology techniques, in particular NMR spectroscopy and X- ray crystallography. Several biophysical techniques (like ITC, MST and BRET) are available to investigate the binding affinities between the small molecules and the corresponding protein target. In cooperation with the Acker-Palmer (P07) group, the effect of the inhibitors on the signaling of the EPH/ephrin system will be investigated in advanced cellular models.

Prof. Dr. Harald Schwalbe
P10: PROJECT-RELATED PUBLICATIONS
- Krishnathas R, Mineev KS, Fourkiotis NK, Touret F, Sideras-Bisdekis C, Taika AC, Gande SL, Linhard V, Sreeramulu S, Lennartz F, Weiss MS, Coutard B, Spyroulias GA, Schwalbe H* (2025) Structure-based rational design of a selective hydrolase inhibitor of the severe acute respiratory syndrome Coronavirus-2 Nsp3 macrodomain. Chem Bio Chem 2: e202500593
- Mineev KS*, Gande SL, Wenzel A, Linhard V, Andrews-Clark H, Witt K, Köster S, Segarra M, McDowell MA, Acker-Palmer A, Schwalbe H* (2025) Structural role of conformational heterogeneity and juxtamembrane regions in the Ephrin A1 interactions with the EphA2 receptor ligand-binding domain. J Mol Biol 437: 169454
- Mineev KS, Hargittay B, Jin J, Catapano C, Dietz MS, Segarra M, Harwardt MS, Richter C, Jonker HRA, Saxena K, Sreeramulu S, Heilemann M, Acker-Palmer A, Schwalbe H (2024) Differential effects of the N-terminal helix of FGF8b on the activity of a small-molecule FGFR inhibitor in cell culture and for the extracellular domain of FGFR3c in solution. FEBS Lett. 20: 2518-2532
- Hargittay B, Mineev KS, Richter C, Sreeramulu S, Jonker HRA, Saxena K, Schwalbe H (2023) NMR resonance assignment of a fibroblast growth factor 8 splicing isoform b. Biomol NMR Assign 1: 135-142
- Tröster A, Jores N, Mineev KS, Sreeramulu S, DiPrima M, Tosato G, Schwalbe H (2023) Targeting EPHA2 with kinase inhibitors in colorectal cancer. Chem Med Chem e202300420
- Neuber C, Tröster A, Löser R, Belter B, Schwalbe H, Pietzsch J (2020) The pyrazolo[3,4-d]pyrimidine-based kinase inhibitor NVP-BHG712: Effects of regioisomers on tumor growth, perfusion, and hypoxia in EPHB4-positive A375 melanoma xenografts. Molecules 25: 511
- DiPrima M, Tröster A, Jores N, Schwalbe H, Tosato G (2020) Receptor tyrosine kinase inhibitors for treatment of protein kinase modulation-responsive disease or disorder E-182-2019-0-PCT-02, patent filed
- DiPrima M, Wang D, Tröster A, Maric D, Terrades-Garcia N, Ha T, Kwak H, Sanchez- Martin D, Kudlinzki D, Schwalbe H, Tosato G (2019) Identification of Eph receptor signaling as a regulator of autophagy and a therapeutic target in colorectal carcinoma, Mol Oncol 13: 2441-2459
- Kappert F, Sreeramulu S, Jonker HRA, Richter C, Rogov VV, Proschak E, Hargittay B, Saxena K, Schwalbe H (2018) Structural characterization of the interaction of the fibroblast growth factor receptor with a small molecule allosteric inhibitor. Chem Eur J 24: 7861-7865
- Tröster A, Heinzlmeir S, Berger BT, Gande SL, Saxena K, Sreeramulu S, Linhard V, Nasiri AH, Bolte M, Müller S, Kuster B, Médard G, Kudlinzki D, Schwalbe H (2018) NVP- BHG712: Effects of regioisomers on the affinity and selectivity towards the EPHrin familiy. Chem Med Chem 13: 1629-1633
