Project 11
Structure and dynamics of the TRPV4 ion channel as a cellular signaling hub
Principle Investigator: Prof. Dr. Ute Hellmich
Research Areas: Structural Biology, Biochemistry
Summary
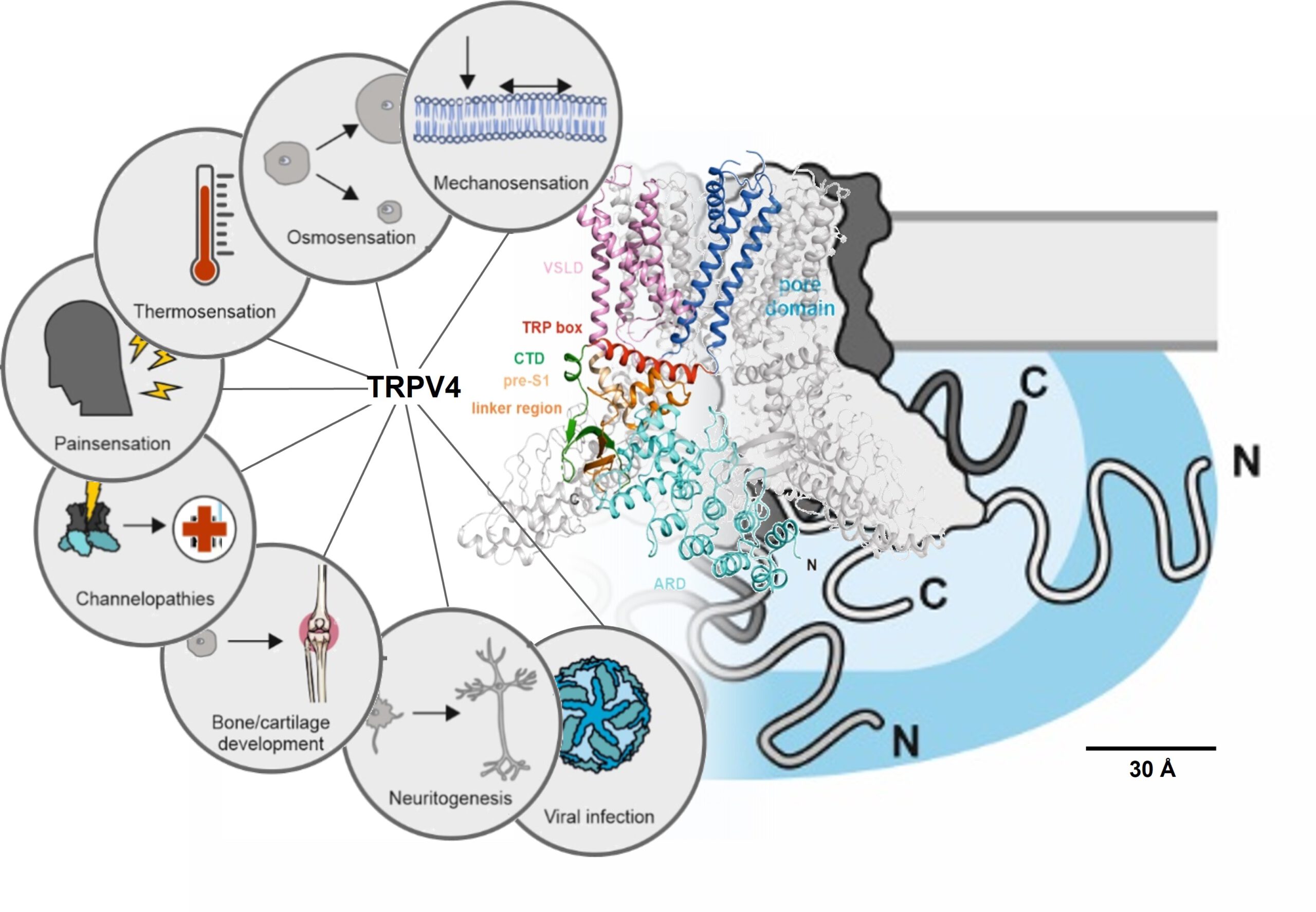
Transient receptor potential (TRP) channels have garnered significant attention as important pharmacological targets in recent years due to their role as cellular polymodal sensory hubs and their involvement in numerous diseases. Nonetheless, their integration into larger cellular signaling networks remains challenging. A detailed molecular description is particularly hampered by the absence of the large intrinsically disordered regions that act as interaction hubs for regulatory proteins and lipids from the currently available TRP channel structures. TRPV4 (vanilloid 4) is a ubiquitously expressed homotetrameric cation channel that plays important roles in temperature, osmo- and mechano-sensation. More than 60 described point mutants lead to severe skeletal and neuronal pathologies, so-called channelopathies. The disease mutations cluster mainly in the channel N-terminus comprised of an ankyrin repeat domain and a 150 amino acid intrinsically disordered region. In this project, we will investigate the conformational ensemble of the TRPV4 N-terminus and the central role of TRPV4 as a cellular signaling hub and coordinator of dynamic, multivalent protein interactions in the plasma membrane using an integrated structural biology and cell biological approach.

Prof. Dr. Ute Hellmich
Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena
Center for Biomolecular Magnetic Resonance, Goethe University, Frankfurt
P11: PROJECT-RELATED PUBLICATIONS
- Berth SH, Vo L, Kwon DH, Grider T, Damayanti YS, Kosmanopoulos G, Fox A, Lau AR, Carr P, Donohue JK, Hoke M, Thomas S, Karam C, Fay AJ, Meltzer E, Crawford TO, Gaudet R, Shy ME, Hellmich UA, Lee SY, Sumner CJ, McCray BA* (2025) Combined clinical, structural and cellular studies discriminate pathogenic and benign TRPV4 variants. Brain 148(2): 564-579
- Schwickert KK, Glitscher M, Bender D, Benz NI, Murra R, Schwickert K, Pfalzgraf S, Schulmeister T, Hellmich UA*, Hildt E* (2024) Zika virus replication is impaired by a selective agonist of the TRPML2 ion channel. Antiviral Res 228: 105940
- Goretzki B, Wiedemann C, McCray B.A., Schäfer SL, Jansen J, Tebbe F, Mitrovic SA, Nöth J, Cabezudo A.C., Donohue JK, Jeffries CM, Steinchen W, Stengel F, Sumner CJ, Hummer G and Hellmich UA (2023) Crosstalk between regulatory elements in disordered TRPV4 N-terminus modulates lipid-dependent channel activity. Nat Commun 14: 4165
- Zhang L, Simonsen C, Zimova L, Wang K, Moparthi L, Gaudet R, Ekhoff M, Nilsson G, Hellmich UA, Vlachova V, Gourdon PE, Zygmunt PM (2022) Cannabinoid non-cannabidiol site modulation of TRPV2 structure and function. Nat Commun 13: 7483
- Goretzki B, Tebbe F, Mitrovic SA, Hellmich UA* (2022) Backbone NMR assignments of the extensive human and chicken TRPV4 N-terminal intrinsically disordered regions as important players in ion channel regulation. Biomol NMR Assign 16: 205-2012
- Wiedemann C, Goretzki B, Merz N, Tebbe F, Schmitt P and Hellmich UA (2022) Extent of intrinsic disorder and NMR chemical shift assignments of the distal N-termini from human TRPV1, TRPV2 and TRPV3 ion channels. Biomol NMR Assign
- Aisenberg WH, McCray BA, Sullivan JM, Diehl E, Devine LR, Bagnell AM, Alevy J, Carr P, Goretzki B, Cole RN, Hellmich UA and Sumner CJ (2022) Multi-ubiquitination of TRPV4 modulates channel activity independent of surface localization. J Biol Chem, 298, 101826.
- Taga A, Peyton M, Goretzki B, Gallagher TG, Ritter A, Harper A, Crawford TO, Hellmich UA, Sumner CJ, McCray BA (2022) TRPV4 mutations associated with mixed neuropathy and skeletal dysplasia phenotypes result in severe gain of ion channel function. Ann Clin Transl Neurol, 9, 375-391.
- Goretzki B, Guhl C, Tebbe F, Harder JM, Hellmich UA (2021) Unstructural biology of TRP ion channels: The role of intrinsically disordered regions for channel function and regulation. J Mol Biol, 166931.
- McCray BA, Diehl E, Sullivan JM, Aisenberg WH, Zaccor NW, Lau AR, Rich D, Goretzki B, Hellmich UA, Lloyd TE and Sumner CJ (2021) Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interaction and impair cytoskeletal regulation. Nat Commun, 12, 1444.
