Project 15
Structure and mechanism of membrane-bound hydrogenases
Principle Investigator: Dr. Bonnie J. Murphy
Research Areas: Structural Biology, Bioenergetics
Summary
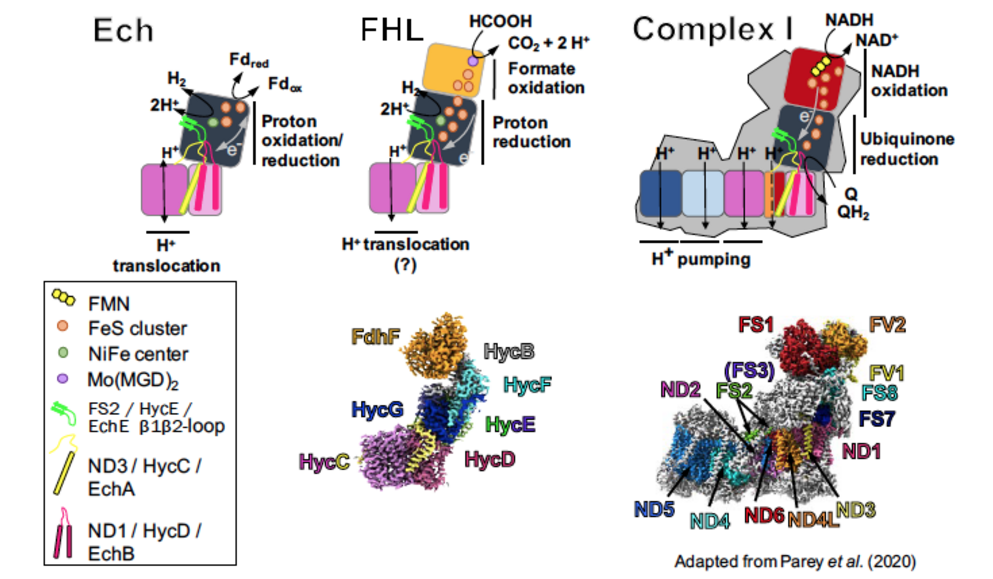
Group IV membrane-bound hydrogenases (MBHs) are members of the Complex I superfamily, catalyzing H2production coupled with ion translocation across a membrane by an unknown mechanism. Using biochemical techniques and anaerobic, redox-controlled single-particle cryo-EM, we will study both the structure and mechanism of two MBHs: the formate-hydrogen lyase (FHL) complex from E. coli, and the energy-converting hydrogenase (Ech) from methanogenic archaea. By solving structures of the complexes under different gas and redox conditions, we will gain insights into the conformational changes that accompany catalytic turnover, and therefore the mechanism of coupling between substrate turnover and ion translocation.

Dr. Bonnie J. Murphy
Max Planck Institute of Biophysics, Frankfurt
P15: PROJECT-RELATED PUBLICATIONS
- Steinhilper R, Höff G, Heider J and Murphy BJ (2022) Structure of the membrane-bound formate hydrogenlyase complex from Escherichia coli. Nat Commun 13: 5395
- Murphy BJ#, Klusch N#, Langer JD, Mills DJ, Yildiz Ö, Kühlbrandt W (2019) Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1 -Fo coupling. Science 364: eaaw9128
- Klusch N#, Murphy BJ#, Mills DJ, Yildiz Ö, Kühlbrandt W (2017) Structural basis of proton translocation and force generation in mitochondrial ATP synthase. eLife 6: e33274
- Kelly CL, Pinske C, Murphy BJ, Parkin A, Armstrong F, Palmer T, Sargent F (2015) Integration of an [FeFe]-hydrogenase into the anaerobic metabolism of Escherichia coli. Biotechnol Rep 8: 94-104
- Murphy BJ, Hidalgo R, Roessler MM, Evans RM, Ash PA, Myers WK, Vincent KA, Armstrong FA (2015) Discovery of dark pH-dependent H(+) migration in a [NiFe]- hydrogenase and its mechanistic relevance: Mobilizing the hydrido ligand of the Ni-C intermediate. J Am Chem Soc 137: 8484-9
- McDowall JS#, Murphy BJ#, Haumann M, Palmer T, Armstrong FA, Sargent F (2014) Bacterial formate hydrogenlyase complex. Proc Natl Acad Sci USA 111: E3948–56
- Bachmeier A, Murphy BJ, Armstrong FA (2014) A multi-heme flavoenzyme as a solar conversion catalyst. J Am Chem Soc 136: 12876-9
- Murphy BJ, Sargent F, Armstrong FA (2014) Transforming an oxygen-tolerant [NiFe] uptake hydrogenase into a proficient, reversible hydrogen producer. Energy Environ Sci 7: 1426-33
- Evans RM, Parkin A, Roessler MM, Murphy BJ, Adamson H, Lukey MJ, Sargent F, Volbeda A, Fontecilla-Camps JC, Armstrong FA (2013) Principles of sustained enzymatic hydrogen oxidation in the presence of oxygen–the crucial influence of high potential Fe-S clusters in the electron relay of [NiFe]-hydrogenases. J Am Chem Soc 135: 2694-707
- Lukey MJ, Parkin A, Roessler MM, Murphy BJ, Harmer J, Palmer T, Sargent F, Armstrong FA (2010) How Escherichia coli is equipped to oxidize hydrogen under different redox conditions. J Biol Chem 285: 3928-3938
